

The oxidation state of alkali metals is always +1, and for alkali earth metals it is +2.The total charge of a molecule or ion is the sum of the charges of each atom.Oxidation state of a single element is zero (this includes the molecules made of a single element as well).The mathematical relationship for this calculation is as follows:įormal charge = (number of valence electrons in neutral atom)- (number of lone pair electrons) – (*bond pair electrons)įigure 02: Oxidation States of Atoms in Different Molecules Tips for the Determination of Oxidation State: We have to divide the bonding electrons equally between the shared atoms.
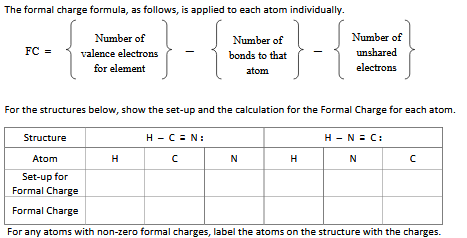
We have to assign nonbonding electrons to the atom in which the non-bonding electrons occur.Here, we need to consider the following requirements as well In this determination of the formal charge, we have to assign the electrons of the molecule to individual atoms. Therefore, when determining the formal charge, we are comparing the number of electrons around a neutral atom and the number of electrons around that atom when it is in a molecule. The formal charge is the charge of an atom in a molecule we calculate assuming that electrons in chemical bonds are shared equally between atoms. Side by Side Comparison – Formal Charge vs Oxidation State in Tabular Form The formal charge determines the number of electrons that occur around an atom of a molecule while oxidation state determines the number of electrons exchanged between atoms during the formation of a molecule. The key difference between formal charge and oxidation state is that formal charge is the charge of an atom in a molecule we calculate assuming that electrons in chemical bonds are shared equally between atoms whereas oxidation state is the number of electrons an atom loses or gains or shares with another atom.įormal charge and oxidation state are different terms though we usually assume they are the same.


 0 kommentar(er)
0 kommentar(er)
